
Frederick Baddour
Researcher IV-Chemistry
Frederick.Baddour@nrel.gov
303-384-6703
 https://orcid.org/0000-0002-5837-5804
https://orcid.org/0000-0002-5837-5804
Research Interests
-
Inorganic synthesis and catalysis
-
Design, synthesis, and characterization of nanomaterials
-
Controlling surface chemistry for selective catalysis
-
Economic modeling of pre-commercial catalyst scale-up
-
Conversion of biomass to fuels and chemicals
Affiliated Research Programs
Education
-
Ph.D., Chemistry, Boston University, 2013
-
B.S., Chemistry, Boston College, 2008
Professional Experience
-
Staff Scientist, National Renewable Energy Laboratory (NREL), National Bioenergy Center (NBC), 2016–present
-
Postdoctoral Researcher, NREL, Chemistry and Nanoscience Center, 2014–2016
Patents
-
"Metal phosphide catalysts and methods for making the same and uses thereof," U.S. Patent Application No. 62/170,906 (2015)
Featured Work
-
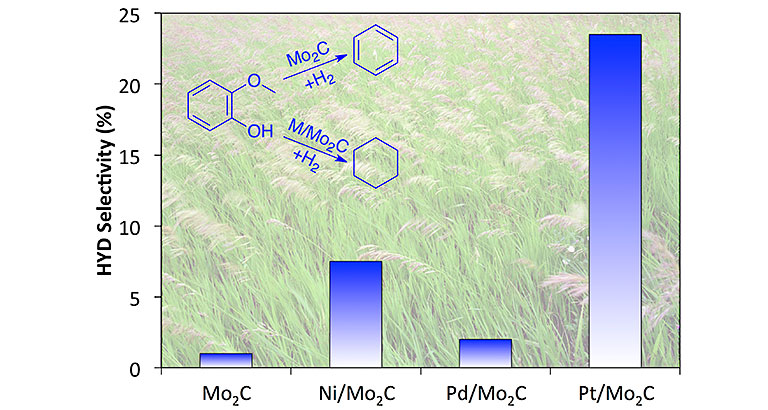
"Late-Transition-Metal-Modified β‐Mo2C Catalysts for Enhanced Hydrogenation during Guaiacol Deoxygenation," ACS Sus. Chem. Eng. (2017)
-
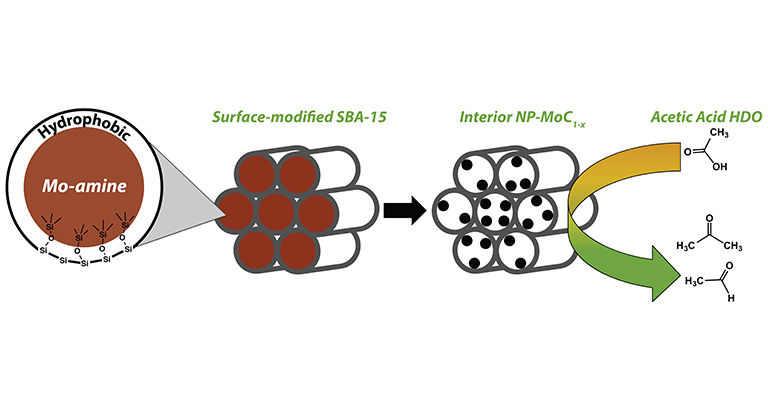
"Synthesis of α-MoC1-x nanoparticles using a surface-modified SBA-15 hard template and determination of structure-function relationships in acetic acid deoxygenation," Angewandte Chemie International Edition (2016)
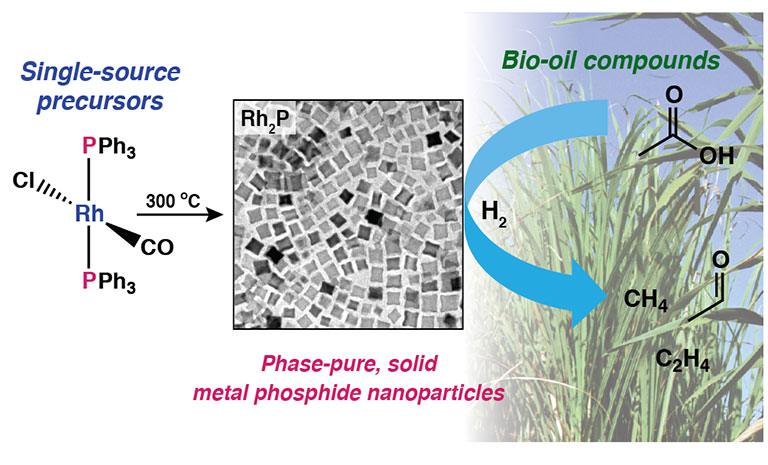
- "A facile molecular precursor route to metal phosphide nanoparticles and their evaluation
as hydrodeoxygenation catalysts," Chemistry of Materials (2016)
Additional Publications
-
"An investigation into support cooperativity for the deoxygenation of guaiacol over nanoparticle Ni and Rh2P," Cat. Sci. Tech. (2017)
-
"High-Throughput Continuous Flow Synthesis of Nickel Nanoparticles for the Catalytic Hydrodeoxygenation of Guaiacol," ACS Sus. Chem. Eng. (2017)
-
"Transitioning rationally designed catalytic materials to real 'working' catalysts produced at commercial scale: Nanoparticle materials," Catalysis (2017)
-
"Pt–Mg, Pt–Ca, and Pt–Zn Lantern Complexes and Metal-Only Donor–Acceptor Interactions," Inorg. Chem. (2017)
-
"Femtosecond Measurements of Size-Dependent Spin Crossover in FeII(pyz)Pt(CN)4 Nanocrystals," J. Phys. Chem. Lett. (2016)
-
"Evaluation of silica-supported metal and metal phosphide nanoparticle catalysts for the hydrodeoxygenation of guaiacol under ex situ catalytic fast pyrolysis conditions," Top. Catal. (2015)
-
"Conceptual Process Design and Techno-Economic Assessment of Ex Situ Catalytic Fast Pyrolysis of Biomass: A Fixed Bed Reactor Implementation Scenario for Future Feasibility," Top. Catal. (2015)
-
"Pt…Pt vs. Pt…S Contacts Between Pt-Containing Heterobimetallic Lantern Complexes," Inorg. Chem. (2013)
-
"Heterobimetallic lantern complexes that couple antiferromagnetically through non-covalent Pt…Pt interactions," Inorg. Chem. (2013)
-
"Antiferromagnetic coupling across a tetrametallic unit through noncovalent interactions," Chem. Sci. (2012)
-
"Platinum(IV)-κ3-terpyridine complexes: synthesis with spectroscopic and structural characterization," Chem. Commun. (2010)
View all NREL publications for Frederick G. Baddour.
Share
