Metabolic Processes, Informatics, and Engineering of Microbial and Plant Systems
NLR researchers seek new understandings of microbial pathways and biophysical processes in microbes and plants, and microbial consortia, to enable scale up of biomass conversion processes from bench to pilot scale.

Enabling the production of cost competitive biofuels, biochemicals, and biomaterials depends on the successful characterization and complementary engineering of plants and microbes. NLR leverages its experience in computational biology with cutting-edge genome editing and metabolic engineering strategies to enable the domestication and redesign of microbes for the conversion of selected or engineered biomass feedstocks to value-added bioproducts at scale.
Microbial Pathways
Microbes hold significant promise as living factories for the synthesis of fuels, chemicals, and materials. To harness their full potential, it is essential to gain a fundamental understanding of their metabolic processes through discovery of metabolic pathways, the regulatory mechanisms that govern these pathways, and the subcellular locations in which the metabolic networks function.
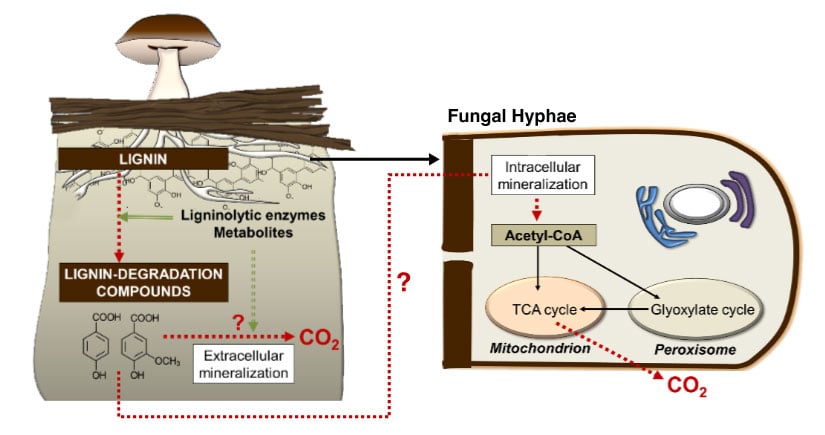
Examples of NLR research projects include elucidating lignin catabolic pathways in white-rot fungi, mapping the extracellular conversion and regulatory mechanisms of secretion of aromatic compounds in outer membrane vesicles released by Pseudomonas putida, and the redesign of microbes to enhance their utilization of electrons.
Many cellular and subcellular processes are carried out by proteins or complexes of proteins. Organisms rely on interactions between proteins to transmit signals, react to stimuli, and control their metabolism. Beneficial protein interactions can increase flux through desired metabolic pathways, channel reaction intermediates to the next enzyme in a pathway or increase the stability of key metabolic enzymes. In cell wall biosynthetic pathways, enzyme complexes conduct template-free synthesis of biopolymers. Some processes involve low-affinity and/or transient interactions between cellular components that are, therefore, difficult to study.
NLR researchers use biophysics-based approaches and structural biology, including single particle cryo-EM, to study and characterize these complexes. We leverage genomic mining, bioinformatics, and artificial intelligence-based models to link genome sequences to macromolecular complex formation and use these predictions in physics-based models (docking and molecular dynamics) to resolve the atomistic basis governing the 3D architecture of interaction/reaction interfaces.
Understanding the formation and function of both stable and transient complexes is essential to conduct informed engineering of microorganisms and plant biomass. Additionally, design principles from these natural complexes can be emulated in natural in vitro systems and in synthetic bio-inspired constructs for cell-free biomanufacturing.
Contact: Davinia Salvachúa Rodriguez
Microbial Consortia
Microbial co-cultures and communities present promising platforms for bioconversion and consolidated bioprocessing (CBP) of lignocellulosic biomass due to their capacity to perform complex functional tasks unachievable by monocultures. Concurrent feedstock deconstruction and conversion by microbial consortia can bypass the necessity for costly, multi-process unit biomass pre-processing, hydrolysis, and upgrading pathways. To this end, NLR researchers are establishing high-efficiency, synthetic microbial consortia that optimize and augment native co-culture systems for CBP, improving both biomass deconstruction and production of diverse platform fuel and chemical intermediates. Advanced analytical chemistry and fermentation engineering approaches are used to define and optimize substrate utilization and product formation in co-culture.
In collaboration, advanced community genome scale metabolic models are being developed to characterize novel community synergies and syntrophies, inform targeted strain and protein engineering strategies for enhanced performance, and establish design rules for next-generation design of microbial consortia. These platforms enable myriad new biotechnological applications.
Contact: Michael Guarnieri
Plant Systems
Plant cell walls account for much of the carbon fixed annually by terrestrial photosynthesis. NLR researchers are combining expertise in polysaccharide structure and function, molecular biology, and computational modeling studies involving molecular dynamics and quantum mechanics simulations to gain mechanistic insight into biopolymer cross-linking in plant cell walls.
Understanding how cell wall components are linked to each other in complex architectures is key to understanding their biological functions and for developing biomaterials with new functionalities. For example, NLR researchers are redesigning poplar wood to reduce the energy inputs needed for subsequent thermal or chemical treatments. Specific cell wall domains involved in cell-cell adhesion contribute disproportionately to biomass structural integrity. Using genetic engineering strategies to gain control over cell-cell adhesion, researchers are developing poplar trees that require less energy to break the wood into small particles for bioconversion.
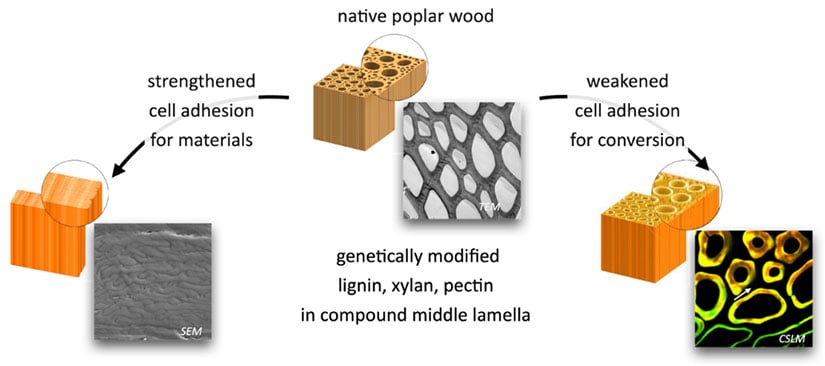
Contact: Bryon Donohoe
Flagship Projects
NLR is a major partner in the Center for Bioenergy Innovation (CBI), an integrated and interdisciplinary research center that aims to deliver technological innovations through the development of process-advantaged feedstocks and consolidated conversion strategies to enable a circular bioeconomy. CBI aims to:
- Develop high-yield, process-advantaged, and plant feedstocks
- Create lower-cost bioconversion processes to produce biofuels and bioproducts
- Develop chemocatalytic conversion processes to produce aviation fuel.
NLR researchers provide deep expertise in:
- The characterization of biomass feedstocks and variants
- The improvement of deconstruction and conversion technologies in consolidated bioprocessing
- High throughput strain and protein engineering
- Separation and valorization of lignin
- Techno-economic and life cycle analyses.
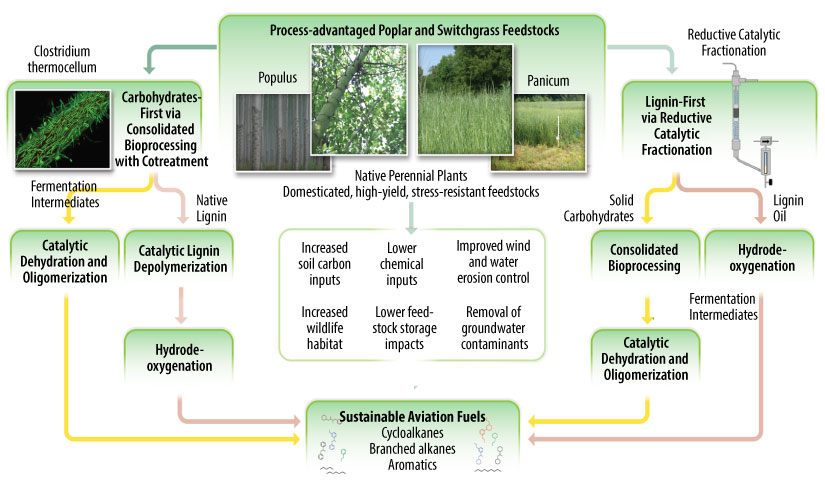
Image from CBI
Contact: Yannick Bomble
The bioconversion of waste and biogenic feedstocks to fuels and chemicals is an integral component of the bioeconomy and has been successfully realized at the laboratory scale. However, bioprocess scale-up has proved to be a considerable challenge, in large part due to uncertainties related to translatability of microbial performance when transitioning to industrial scale.
In contrast to the well-mixed cultivations at the laboratory scale, large-scale cultivations face a series of engineering hurdles driven by nonuniform mixing, which results in pH, gas composition, temperature, and nutrient gradients in bioreactors. These gradients generate "microenvironments" that impact microbial performance in an unpredictable manner, decreasing bioconversion efficiency and increasing manufacturing costs, which can prevent or substantially delay the implementation of a new bioprocess. Unless the capability to predict microbial performance in large-scale bioreactors can be realized, many bioconversion pathways will not come to fruition in the bioeconomy.
This project aims to establish a pipeline to address the adequacy of a microbe at the beginning of the innovation cycle and predict its performance for scale-up to help mitigate risks during the development of new bioprocesses. This pipeline could enable faster, cost-effective, and more reliable bioprocess commercialization with profound impact for biotechnology-related industries.
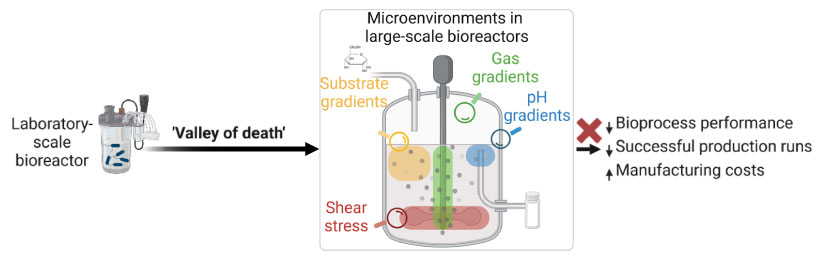
The transition between laboratory- and industrial-scale cultivations represents a “valley of death” in bioprocessing, where uncertainties arise regarding the predictability for microbial performance across scales.
Contact: Davinia Salvachúa Rodriguez
NLR develops and generates strains of cyanobacteria that affect specific aspects of carbon, nitrogen, phosphorus, and energy metabolism together with phenotyping tools that measure and model key parameters in photosynthesis and metabolism. Some cyanobacteria are capable of fixing nitrogen gas into nitrogen compounds that support metabolism. Such cyanobacteria have potential to produce biofertilizer with beneficial impacts for agriculture. NLR has developed novel chemistry to enable secretion of nitrogenous molecules and is introducing this capability into nitrogen-fixing cyanobacteria to increase biofertilizer production and probe the molecular mechanisms that control biological nitrogen fixation and metabolism.
Contact: Jianping Yu
Glycosyltransferases (GTs) catalyze the formation of glycosidic linkages to produce complex carbohydrates. NLR researchers are taking a multidisciplinary, high-throughput biochemical and computational biology approaches to study biosynthetic processes involving GT complexes in duckweed, a promising bioenergy crop. The role of enzymatic microenvironments is being assessed using proteomics and computational biology approach, and the data are used to populate a deep-learning framework to predict plant GT function.
Contact: Yannick Bomble
NLR, in association with structural biology researchers at SLAC National Accelerator Laboratory, is deploying molecular simulations of protein-ligand complexes at scale to streamline drug-discovery pipelines. NLR is spearheading the development of advanced molecular docking and molecular dynamics simulations of protein-ligand and protein-protein complexes, which will provide robust biophysical methodologies to enable insight into binding mechanisms and aid the development of novel drug molecules to combat future pandemics.
Contact: Yannick Bomble
Significance and Impacts
By understanding protein machines, and biosynthetic pathways in microbes, plants, and microbial communities, NLR researchers are paving the way for the bioconversion of waste carbon sources to create economic value.
Contact
Share
Last Updated Dec. 7, 2025
