Organic and Molecular Semiconductors
NLR aims to acquire a fundamental understanding of interfacial photoinduced electron transfer processes for semiconductors at molecular, nanoscale, and organic interfaces.
Specific organic systems that are examined include conjugated polymers, single-walled carbon nanotubes, molecular films, and organic colloidal nanoparticles.
Doomed Charge-Transfer States Populated by Energy Transfer
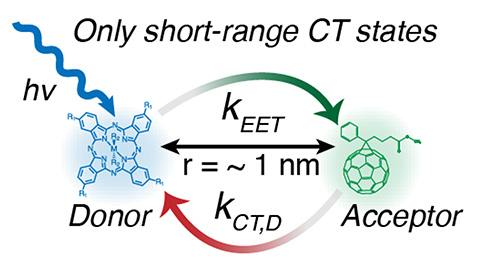 Missing Excitons: How Energy Transfer Competes with Free Charge Generation in Dilute-Donor/Acceptor
Systems, ACS Energy Letters (2024)
Missing Excitons: How Energy Transfer Competes with Free Charge Generation in Dilute-Donor/Acceptor
Systems, ACS Energy Letters (2024)We find that the yield of free charges in a model organic photovoltaic (OPV) system depends on the initial excited-state energy in a surprising way. High-energy (blue) absorbing donor molecules exhibit significantly reduced yield relative to low-energy (red) ones due to a very competitive energy transfer process.
Research Details
- Yield of free charges detected using microwave spectroscopy
- Charge-transfer (CT) state tracked using transient absorption spectroscopy
- Strong coupling between sensitizer and host identified by spectral line broadening and shifting
- High carrier yields can result in these systems when the host is excited, rather than the blue sensitizer
Significance and Impact
The adage that excitations in an OPV solar cell diffuse to the interface between the donor and acceptor and dissociate into an intermediate CT state is proving to be false. Instead, long-range electron transfer events are required to form free charge, and the exciton need not be immediately adjacent to the interface.
Partner
University of Colorado Boulder
Excitons Just Need a Little Space To Separate
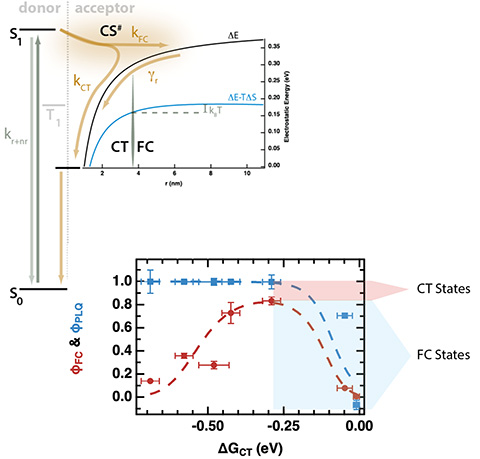
Short and Long-Range Electron Transfer Compete to Determine Free-Charge Yield in Organic Semiconductors, Material Horizons (2021)
We have demonstrated that the yield of free charges from photoinduced electron transfer (PET) in electron donor-sensitized fullerene (PCBM) films is dependent upon the driving force, exhibiting clear inverted behavior even in the presence of extended fullerene aggregates—consistent with an electron-transfer model based on the Marcus rate equation for PET.
Research Details
- A series of guest molecules were identified to control the driving force for photoinduced electron transfer and selective photoexcitation.
- Photoinduced electron transfer from the guest to the host PCBM was monitored using time-resolved microwave conductivity (TRMC) and photoluminescence quenching (PLQ).
- The new distributed-range electron transfer model was successfully used to analyze globally the data from TRMC and PLQ experiments.
Significance and Impact
This new model unifies solution and solid-phase electron transfer theory and contradicts current opinion in the field of organic photovoltaics that the CT state is the precursor to free charges.
Partners
University of Colorado Boulder
Tailored Aggregation of Tetracene for Solution Phase Singlet Fission
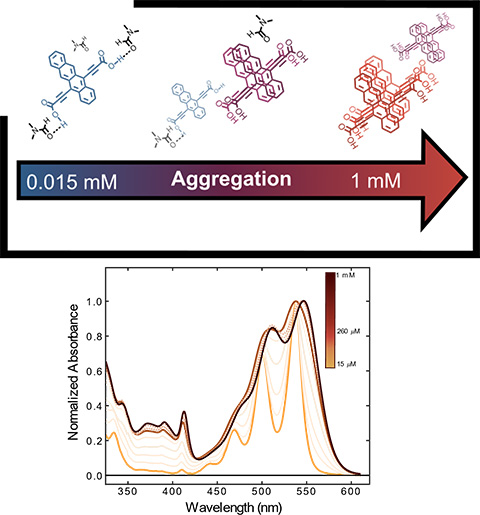 Tetracene Diacid Aggregates for Directing Energy Flow Toward Triplet Pairs, Journal of the American Chemical Society (2024)
Tetracene Diacid Aggregates for Directing Energy Flow Toward Triplet Pairs, Journal of the American Chemical Society (2024)A comprehensive investigation of the solution-phase photophysics of a tetracene bis-carboxylic acid and its related methyl ester reveals the role of hydrogen bonding and π-π interactions in driving molecular aggregation.
Research Details
- Synthesis of a novel tetracene with bis-carboxylic acid functionality was researched.
- Steady state and transient absorption spectroscopy were utilized to characterize the photophysics of discrete tetracene aggregates in solution.
- Electronic structure and density functional theory model the intermolecular orientation of tetracene subunits and accurately predicts their ground-state absorption behavior.
Significance and Impact
Traditionally, materials that exhibit singlet fission rely on carefully designed solid state packing or covalent linkages. In this work, we unveil a new tetracene derivative that organizes into discrete aggregates based on the solvent environment and diacid’s concentration in solution.
Partner
University of Colorado BoulderPast Research Highlights
Reconciling the Driving Force and the Barrier to Charge Separation in Donor-Nonfullerene Acceptor Films, ACS Energy Letters (2021)
We discovered microwave conductivity measurements facilitate a candid assessment of how driving force calculations are performed. They also highlight how underestimated driving force values lead to precarious interpretations of charge separation dynamics in donor-nonfullerene acceptor films relevant to record-setting organic photovoltaics (OPVs).
Research Details
- Solution phase electrochemistry enabled quantitative driving force calculations for all-small-molecule films.
- Time-resolved microwave conductivity measurements observed dramatic dependence of charge yields on driving for sensitized and blended films.
- Photoluminescence quenching and lifetime measurements corroborated microwave data.
Significance and Impact
The research addresses the pitfalls for popular driving force calculation methods that obscure the subtle dependence of charge separation efficiency on driving force. In our call for standardized methods, we contributed to a unifying movement for OPVs at a time where coherent progress in devices and fundamental understanding is crucial.
Partner
We have successfully identified electron acceptors capable of dissociating triplet excitons from singlet fission in pentacene films. However, even at the optimal driving force, the rate constant for electron transfer is surprisingly small.
Research Details
- Successfully dissociated triplets from singlet fission in pentacene into free carriers
- Identified and found optimum driving force to follow Marcus formulation
- Discovered rate constant for photoinduced electron transfer is 5–6 orders of magnitude slower than for singlet states
Significance and Impact
Slow dissociation process contrasts with singlet excitons, opening up other competing pathways that may prove to be an obstacle to the design of efficient singlet fission solar cells that are based on a direct dissociation process.
Partners
We have demonstrated heterojunctions between transition metal dichalcogenides and single-walled carbon nanotube with exceptionally long, microsecond timescale, charge separation following sub-picosecond interfacial charge transfer. These carrier lifetimes are orders of magnitude longer-lived than in other monolayer transition metal dichalcogenide heterojunctions.
Research Details
- Monolayer molybdenum disulfide (MoS2) grown by chemical vapor deposition
- Highly enriched (6,5) semiconducting single-walled carbon nanotubes
- Ultra-fast time-resolved spectroscopy
Significance and Impact
Long-lived separated charge carriers are a prerequisite for efficiently converting photon energy to electricity or fuels in solar energy harvesting devices. With this research, we have counteracted ultrafast excited state decay in transition metal dichalcogenide monolayers by demonstrating that heterojunctions between molybdenum disulfide and single-walled carbon nanotubes enable remarkably long carrier lifetimes in the microsecond time range.
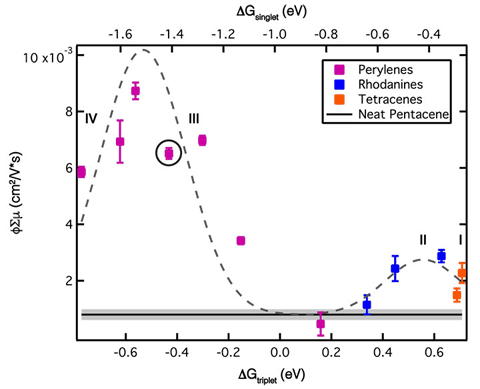
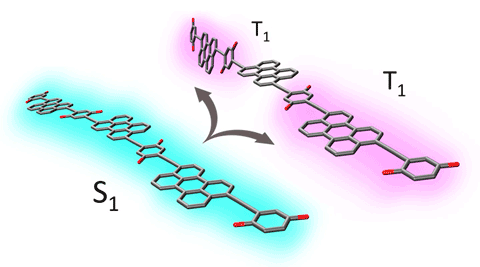
We discovered two triplet excitons born from one photon absorption event in chains of molecular absorbers are formed with no loss of energy and with lifetimes into the microsecond regime through the involvement of spatial separation and dynamic geometric isolation.
Research Details
- Designed and synthesized perylene oligomers with varying numbers of chromophores that are strongly coupled through bridges
- Found significant triplet yield only in trimer and longer structures
- Spectroscopy and calculations showed that oligomers undergo planarization in the singlet excited state before singlet fission but that torsional motions subsequently isolate triplets
Significance and Impact
The design of strongly coupled yet flexible chromophores demonstrates a new paradigm for producing useful high-energy, triplet excitons in molecular architectures. They have potential as components in photovoltaics or photocatalysis schemes that previously were plagued by significant heat loss, poor efficiency of triplet separation, or short-lived triplet excitons.
Contact
Share
Last Updated Dec. 6, 2025