Photo and Electrochemical Redox Systems
NLR seeks to understand how chemical and physical structure affects the thermodynamics and kinetics of charge separation and redox chemistry in photo(electro)chemical systems.
We do this by using spectroscopic probes to understand charge separation lifetimes and charge transfer rates in bulk semiconductor and/or dark electrodes, quantum-confined semiconductors such as transition metal dichalcogenides (TDMCs), and strongly coupled systems such as catalysts strongly coupled to excitons in a semiconductor or plasmonically coupled systems.
Model System to Understand Solar Fuels Assemblies
Silicon Nanocrystal Hybrid Photocatalysts as Models to Understand Solar Fuels Producing Assemblies, Sustainable Energy & Fuels (2024)
A model hybrid molecular catalyst-semiconductor photoelectrochemical assembly retains the carbon dioxide (CO2) reduction activity to carbon monoxide of the native catalyst despite minimal electronic coupling between the silicon semiconductor and electrocatalyst.
Research Details
- Silicon nanocrystals decorated with Lehn-type molecular CO2 reduction catalysts were studied using electrochemical, spectroelectrochemical, and spectroscopic probes.
- These studies unveiled the CO2 reduction activity and semiconductor-catalyst energetics.
Significance and Impact
Surface tethering of catalysts to semiconductor surfaces can have beneficial impact on catalyst durability. The energetic match between the semiconductor and catalyst is an essential design component of hybrid molecular catalyst-semiconductor systems.
Preventing Ammonia Loss with Membrane Selection
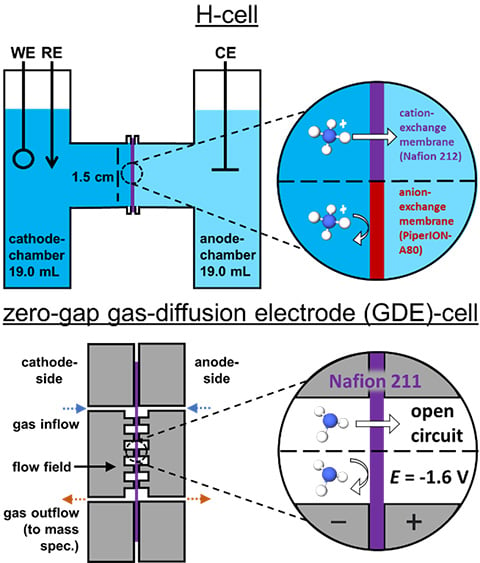
Membranes Matter: Preventing Ammonia Crossover during Electrochemical Ammonia Synthesis, ACS Appl. Energy Mater (2024)
The electrochemical nitrogen and nitrate reduction reactions (E-NRR and E-NO3RR) promise to provide decentralized and fossil-fuel-free ammonia (NH3) synthesis.
Research Details
- Regarding H-cell configuration tests:
- Commonly used Nafion 212 readily crossed ammonium ion (NH4+) during a 6-hour trial.
- Anion exchange membranes (AEMs) show greatly reduced or negligible NH4+ crossover over a 6-hour trial.
- AEM PiperION-A80 is recommended.
- Negligible crossover of NH4+ shown in acidic and neutral pH electrolyte.
- Nafion 212 and 211 shows ammonia (NH3) crossover without applied potential
- Applying -1.6 V across the cell prevents NH3 crossover of Nafion 211.
Significance and Impact
Industrial production of NH3 is currently energy intensive and has a large C-footprint. As the community explores electrocatalysts and alternative electrochemical routes for NH3 generation, it is critical that the test setup correctly accounts for NH3 generated. This work recommends certain membranes based on the type of setup and experimental conditions used, as well as experimental parameters for electrolyzers, which operate intermittently.
Activating Nitrogen for Electroreduction Via an Electrified 2D-Transition Metal Dichalcogenide Catalyst
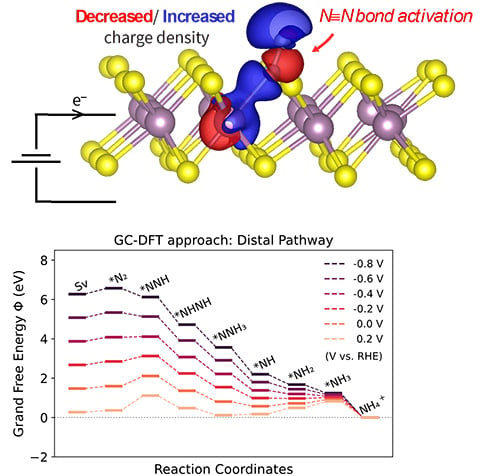
Employing state-of-the-art Grand Canonical Density Functional Theory (GC-DFT) methods, we investigate the impact of the molybdenum disulfide catalyst’s electrified interface on nitrogen (N2) reduction.
Research Details
- Reductive potentials activate N2 by controlling its backbonding strength.
Similar trends are observed for carbon monoxide, suggesting that this mechanism may be broadly relevant towards electrochemistries involving backbonded adsorbates.
A careful balance must be struck between favorability of N2 adsorption and hydrogenation.
GC-DFT facilitates modeling all these phenomena that can have important implications in predicting electrocatalyst activity and selectivity.
Significance and Impact
Our finding that chemisorption steps can be potentially dependent has important implications for how catalyst selectivity is determined theoretically. Additionally, our calculated pathways highlight a trade-off between N2 adsorption and subsequent hydrogenation steps being thermodynamically favorable. A design principle could be to target catalysts in which N2 remains favorably adsorbed under the potentials necessary to drive electroreduction.
Past Research Highlights
Carbon Dioxide and Nitrogen Reduction Reactions Using 2D TMDC and MXene Catalysts, Energy & Environmental Science (2021)
With an eye towards next-generation technologies with lower energetic costs and greenhouse gas emissions, we addressed (photo)electrocatalytic and photocatalytic trends, and lessons learned to improve 2D transition metal dichalcogenide (TMDC) and transition metal nitrides/carbide (MXene) catalysts for carbon dioxide and nitrogen reduction reactions.
Research Details
- Explored 2D TMDC and MXene materials for carbon dioxide and nitrogen reduction reactions
- Addressed how defects, phases, edge sites, interfaces, doping, and functional groups can be engineered to improve catalytic performance
- Provided our perspective on advancing these two material classes within (photo)electrocatalytic and photocatalytic setups before they could be considered industrially viable catalysts.
Significance and Impact
Converting carbon dioxide into useful C-based products and revolutionizing industrial ammonia generation are two scientific grand challenges with the potential to solve critical global energy and greenhouse gas threats. We explored (photo)electrocatalytic and photocatalytic methods as potential solutions by addressing 2D TMDC and MXene catalysts.
Transient absorbance measurements showed that molybdenum diselenide (MoSe2) and tungsten diselenide (WSe2) decorated with gold nanoparticles show hot-hole transfer from the gold to the transition metal dichalcogenide, producing a charge-separated state.
Research Details
- Gold nanoparticle-decorated mono- to few-layer transition-metal dichalcogenides nanosheets were prepared using liquid-phase exfoliation and direct growth of gold nanoparticles from solution.
- Thin films were deposited by blade coating.
- Photogenerated carrier dynamics in these nanosheets were studied using transient absorbance.
Significance and Impact
Efficient injection of hot holes at the metal-semiconductor interface represents an important advance in harnessing plasmonic dissipation pathways to generate long-lived carriers suited for solar-driven light harvesting and catalysis.
Partner
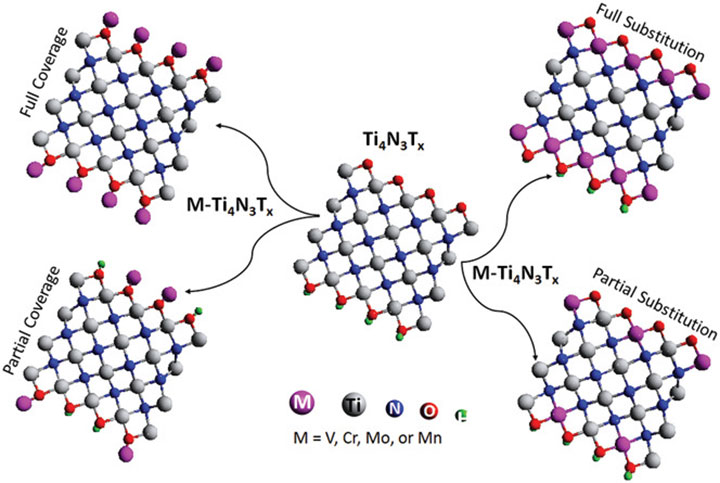
Basal Plane Hydrogen Evolution Activity from Mixed Metal Nitride MXenes Measured by Scanning Electrochemical Microscopy, Advanced Functional Materials (2020)
2D mixed-metal nitride MXenes M-Ti4N3Tx (M = V, Cr, Mo, Mn) were synthesized by a novel solution-based method and characterized via scanning electrochemical microscopy.
Research Details
Hydrogen evolution reaction activity results from the transition metal ions comprising the outer layer of the basal plane in MXenes, which is distinct from other 2D materials such as transition metal dichalcogenides where the catalytically active transition metals are only exposed on the edges.
There is much debate in the community surrounding whether MXenes are intrinsically metals or can exhibit semiconducting properties, and this report conclusively demonstrates the latter.
Significance and Impact
Scanning electrochemical microscopy data showed the first experimental evidence that MXenes exhibit hydrogen evolution reactionactivity from their basal planes, the large area "flat" part of the 2D structure. Additionally, scanning electrochemical microscopy provided evidence for semiconducting behavior, which represents only a handful of reports showing that MXenes can be semiconducting in addition to metallic.
Partner
Contact
Share
Last Updated Dec. 6, 2025