NREL Research Dives Deeper Into the Mysteries of Energy Control in Electron-Bifurcating Enzymes
Understanding the Catalytic Mechanism of This Unusual Class of Enzymes May Be a Precursor to More Efficient Catalysis in the Production of Fuels and Chemicals

This team of researchers has made progress toward mapping the energy landscape of flavin-based electron bifurcation reactions. Standing in front of NREL’s Research and Innovation Laboratory are (left to right) Seth Wiley, Saad Imran, Cara Lubner, Gregory Vansuch, Michael Dawson, and Jonathan Humphreys. Photo by Joe DelNero, NREL
In 2019, National Renewable Energy Laboratory (NREL) senior scientist Cara Lubner had just received a U.S. Department of Energy Office of Science Early Career Research Program Award. Since then, Lubner and her team have made considerable progress toward mapping the energy landscape of an enzymatic reaction.
The $2.5 million research project, titled "Elucidating the Mechanistic Determinants of Flavin-Based Electron Bifurcation," sought to build upon previous discoveries by the research team on mechanisms that flavin-based enzymes use to generate higher energy products during their metabolic reactions. This mechanism is known as flavin-based electron bifurcation (FBEB), which is a recently discovered fundamental mechanism of energy conservation.
Flavin-based bifurcating enzymes are biocatalysts that have evolved to perform efficient metabolic reactions in harsh environments with low nutrients. FBEB reactions are a much more efficient form of catalysis than using microbes to convert biofeedstocks into fuel.
Now Lubner and her team understand the specific mechanism of how flavin-based biocatalysts transform electrochemical potential into chemical bonds and achieve optimal energy conservation.
"Building upon our previous research, our questions included how the properties of the flavin bifurcating site are tuned by the nearby electron donors and acceptors and how this site controls the two electrons without energy losses," Lubner said. "We have now discovered the answers to these questions, which has allowed us to map out the complete physical and electronic landscape of flavin electron bifurcating sites."
Rendering the Landscape of the 10 Picosecond Enzymatic Reaction
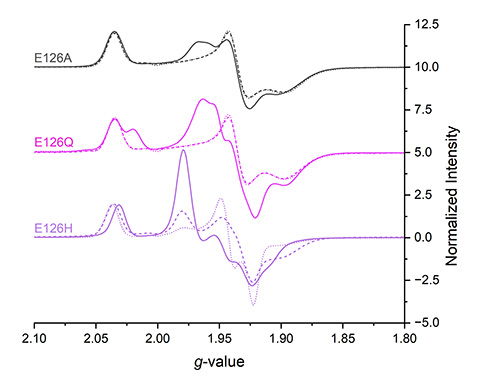
Electrons move incredibly fast, moving between enzyme cofactors in as little as 10 picoseconds (a picosecond is one trillionth of a second). Lubner’s team had to develop ultrafast techniques in the lab to see these processes. Such techniques are akin to atomic-level time-lapse photography using a laser. They paired these techniques with electron paramagnetic resonance spectroscopy to peek into the electron's environment.
With the enzymatic reaction captured for human observation, the team began the process of defining the energy landscape of the reaction by mapping the environment and how the flavin controls movement of energy along the electron transfer pathways.
Energy naturally flows “downhill” from a higher state to a lower one. In this enzymatic reaction there are two electron pathways, one that travels to a higher energy product and another to a lower energy product. The team has been exploring why the higher energy pathway is positioned closer to the flavin versus the lower energy pathway. An electron pathway with a shorter distance means less time and therefore less energy used for electron transfer.
In addition to the location of the electron pathways, the enzyme exhibits independent control over the two electrons. Two-electron-chemistry flavins typically send two electrons downhill to the same place. This is not necessarily efficient but is easier to do. Lubner’s team discovered that this enzyme does one-electron-chemistry twice, on an energetically uphill and an energetically downhill path, which happens to be a super-efficient method of electron transfer.
"Spatially, the arrangement of the energy flow pathways during the reaction provided clues on how the flavin is controlling the electrons on each pathway. The enzyme has found a way to adjust the energy levels of the flavin so it can form a high-energy intermediate. By first sending one electron on the downhill path, it generates a species that has enough energy to travel along the higher energy pathway," Lubner said. "Our research explains the biochemical and physiological observations that there is strict partitioning of one electron down each pathway.”
This fundamental knowledge sets the stage for advancing synthetic catalysts that can generate and control high-energy intermediates to efficiently drive challenging chemistry.

Tuning the Enzyme for More Efficient Catalysis
As a result of this research, Lubner’s team has begun to experiment with engineering the enzyme to modify its properties. The team recently discovered that by making an adjustment to the way the enzyme holds onto an internal cofactor molecule, they can change the energy landscape.
Lubner said making a change to the way the protein binds to its cofactor seems to have disrupted the strict partitioning of energy at the flavin site, favoring energy transfer along just one of the pathways. The team hypothesizes that the change affects the coupling of the two pathways in yet unknown ways and is the subject of current studies. This work has been demonstrated in the lab, but now the team wants to test to see if this engineered enzyme can work inside the cell.
Using the knowledge gained through this work, energetically uphill reaction pathways in cells could be co-opted to produce industrially relevant compounds.
“It’s promising that we can take some of the knowledge we’ve gained from this Early Career Award research to test new hypotheses about how this enzyme integrates with metabolic networks,” Lubner said. “Possible benefits of these metabolically engineered enzymes could be to drive more efficient production of ethanol and butanol or other chemicals used in sustainable aviation fuels.”
Cara Lubner was also part of a group of researchers (including NREL’s Paul King and David Mulder) who won the prestigious Royal Society of Chemistry 2023 Faraday Horizon Prize for electron bifurcation research.
Read more about NREL’s basic energy sciences research or the work supported by the U.S. Department of Energy Office of Science.
Last Updated May 28, 2025
